(Was Model 6261)
Standard Features:
- Sterile-Design for Purity Hygienic Applications
- SF4 Surface Finish (15 Ra Max) Electro-polished
- Machine Finish 220 Grit
- All Gaskets Meet USP Class VI, are FDA Compliant and are Fully Traceable
- Suitable for SIP/CIP, Sterilizing and Autoclaving
Connection Sizes:
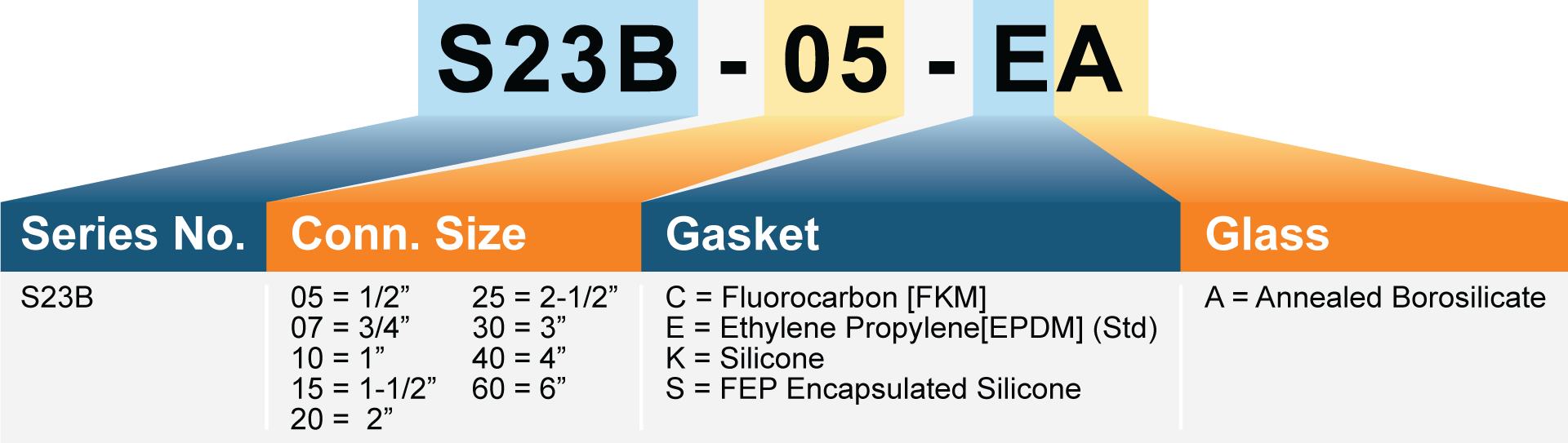
- 1/2″ to 6″ Tri-Clamp (Per ASME-BPE
Standard Wetted Materials:
- Body: 316LSS
- Internal Gaskets: Ethylene Propylene (EPDM), Fluorocarbon(FKM) or FEP Encapsulated Silicone (All gaskets are FDA Compliant, USP Class VI Compliant)
- Glass: Borosilicate Tube
Standard Non-Wetted Materials:
- Not Applicable
Options:
- Fluorocarbon [FKM], Silicone FEP encapsulated Silicone Gaskets
- Orbital Ends
Application Notes:
- Pharmaceutical Applications: Recommended
- Steam Service: Not acceptable
- Vacuum Service: Acceptable
- Gaseous Service: Not designed for lightweight gases
- Corrosion: Before ordering, verify all materials of our sight flow indicators are compatible with the service media(s), intermittently used cleaners, the environment, and cleaning processes in conjunction with the temperatures and pressures of the application(s) and future applications. Ernst Instruments does not make chemical compatibility recommendations. Compatibility is the responsibility of the user.
Replacement Parts:
- Please contact our sales department
Alternate Equipment:
- None